A flow cytometer is a cell detection device capable of detecting and counting a laser beam passing through a single-cell liquid stream. Since the cell marker tracer used in the detection is a fluorescent marker, it is used for separation. Flow cytometry for identifying cells, known as fluorescence-activated cell sorters, is a powerful tool for isolating and identifying cell populations and subpopulations.
experimental method
- Flow cytometry
| Principle of experimental method | The use of flow cytometry is generally divided into three steps. The first is the pre-instrument stage, including reagent preparation, cell preparation, and fluorescent staining of cells; second, the flow cytometry stage, mainly the operation of the instrument; and third, the result analysis stage. |
|---|---|
| Experimental Materials | Leukocyte in peripheral blood of lymphocytes |
| Reagents, kits | FACS buffer PBS sodium azide solution instrument buffer FACS cleaning solution FACS cleaning solution |
| Instruments, consumables | Flow cytometry |
| Experimental procedure | one, Preparation of cells and reagents 1. Cell sample: white blood cells derived from lymphocytes or peripheral blood. 2. Fluorescently labeled monoclonal antibodies: Commonly used are monoclonal antibodies labeled with FITC (fluorescein-isothiocyanate) and PE (phycoerythrin). 3. Blocking antibody: anti-Fc receptor antibody 4. FACS buffer: 1×PBS 950 ml FCS 40 ml 10% sodium azide solution 10 ml 5. Instrument buffer (FACS-flow): provided by the instrument manufacturer 6. FACS clean solution (FACS clean) and FACS rinse (FACS rinse): supplied by the instrument manufacturer. Second, the operation steps 1. Preparation of single cell suspension: Centrifuge 1 × 10 5 ~ 1 × 10 7 cells in a 1.5 ml centrifuge tube at 3000 r / min for 5 minutes, discard the supernatant; add 100 μl of FACS buffer to suspend the cells. 2. Block cell surface Fc receptor: Add 0.5 μl (0.5 mg/ml) of Fc receptor antibody in step 1 and bath for 3 minutes. 3. Binding of the cells to the fluorescent antibody: Add 1 μl (0.5 mg/ml) of the fluorescent antibody in step 2 and let the bath stand for 30 minutes. 4. Wash the cells to remove the free fluorescent antibody: Add 350 μl of FACS buffer in step 3. Mix gently, centrifuge at 3000 r/min for 5 minutes, discard the supernatant, and repeat step (4) twice. 5. Pretreatment before loading: Add 100 μl of instrument buffer to the cell pellet obtained in step (4), gently mix the suspension cells, and transfer the cell suspension into a FACS tube for instrument detection and analysis. Third, the operation of the instrument Take the FACSCaliburTM (BD Biosciences) instrument as an example. The instrument is mainly divided into a host part (including laser activation source, jet, ray detector) and a computer part (CELLQuest softwere). The operation of the instrument is divided into pre-use preparation, in-use operation, and post-use cleaning. 1. Preparation before use (1) Turn on the power: including the system power and the main part of the power supply. (2) Check the liquid supply tank and waste liquid tank: The liquid supply tank should contain enough instrument buffer, and the waste liquid tank should be cleaned after the last use. (3) The machine is placed in a negative sub-state to allow the cell suspension to be drawn into the suction port of the machine. (4) When the machine is in the applicable state, the indicator will turn green. 2. Operation in use (1) Computer Part - Using the CELLQuest Program System Software: 1 Set the number of cells to be detected: generally 10,000 to 20,000 cells. 2 Name this experiment: generally contains the user's name and the date of the experiment. 3 Turn on the counting section: Show the number of cells entering and the time required to detect a certain amount of cells. 4 Connect the microcomputer to the host: control pre-detection and actual detection during detection. 5 host settings: generally set the conditions suitable for measuring lymphocytes, including the detector voltage. The voltage of the detector is the range of fluorescence density that can be detected by the nulling photomultiplier tube. 6 Select the wavelength and expression of the assay (plot): If a fluorescent antibody is used, a histogram is generally selected; if two fluorescent antibodies are used, a dot plot or a contour map is often selected. Contour plot) Numerical Description of CD69 Molecular Expression Detection Histogram  b.abti-CD3 (2 ng/ml) test results 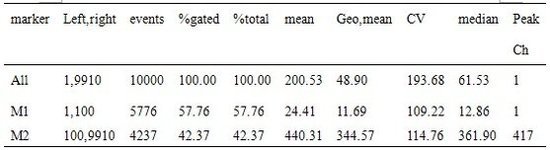 c.abti-CD3 (10 ng/ml) test results  Plot 2D results of CD4 and CD8 cells  Different phosphors require different excitation wavelengths, see table below  (2) Inhalation of cells: Place the FACS measuring tube containing the cells into the suction hole of the machine. The sample is pre-tested and then tested. The measured speed (low, medium or high) can be selected depending on the concentration of the cells. All data will be automatically saved to the PC. 3. Cleaning after use After all samples have been measured, the instrument should be cleaned. (1) Place the FACS tube containing the FACS cleaning solution into the suction tube hole of the machine and inhale for 1 minute at high speed. (2) Place the FACS tube containing the FACS cleaning solution into the suction tube hole of the machine and inhale for 1 minute at high speed. (3) Place the FACS tube with FACS cleaning solution in the suction tube hole and inhale for 5 minutes at high speed. (4) Place the FACS tube containing the FACS cleaning solution in the suction tube hole and inhale for 5 minutes at high speed. (5) Finally, after placing a FACS tube with double distilled water in the suction tube hole of the machine, turn off the machine. (6) Drain the waste liquid in the waste tank and clean the waste tank. |
| Precautions | 1. Reduce non-specific fluorescent staining (1) Cell activity and state: The flow cytometer can detect not only the fluorescence on the cell surface but also the fluorescence inside the cell. Therefore, if you want to detect the molecules on the cell surface, you must ensure the activity of the cells, and should keep the cells as static as possible, usually at 4 degrees. Otherwise, fluorescent antibodies enter dead cells, producing non-specific results. (2) The use of blocking antibodies is essential because most immune cell surfaces express Fc-R. After the cells interact with the antibody, they must be washed 2 to 3 times with FACS buffer to remove free antibodies. 2. FACS is most commonly used in single staining, and FITC-labeled antibody fluorescence is relatively stable and affordable. 3. If you want to detect more than two molecules at the same time, be sure to select different wavelengths of fluorescently labeled antibodies. |
RICTRON Industrial Co., Ltd is a hight-tech OEM/ODM enterprise, we are specialized to produce the products such as fire smoke alarm, carbon monoxiede alarm, gas alarm, water alarm, smoke&co or gas&co combined alarms, and other products of R&D, manufactring and sales since 2008.
We continually strive to maintain our unwavering reputation through our exceptional product quality and customer service. We not only offer a complete line of superior quality products, but also work with our customers to develop new formulas and address the burgeoning market. So, we get so many goods response from our global customers. Welcome your exlusive customization of OEM and ODM, we will grow together and create brillant with you.
Gas Detector,Gas Sensor,Gas Leak Detector,Lpg Gas Leakage Detector
Rictron Industrial Co., Ltd , https://www.szrictron.com